[ad_1]
CRISPR, the gene-modifying know-how that has revolutionized organic analysis, is lastly readily available as a health care remedy with regulatory approval. On December 8 the U.S. Meals and Drug Administration authorized the 1st CRISPR remedy for sickle mobile sickness.
The therapy, called exa-cel and designed by the companies Vertex and CRISPR Therapeutics, edits a gene associated in purple blood cell condition and function. It appears to functionally overcome the ailment for at least a single year. The FDA’s final decision can make the U.S. the next nation to approve a CRISPR treatment, next exa-cel’s acceptance for sickle cell disease in the U.K. in November.
Scientifically talking, exa-cel is “an incredible asset to have,” suggests Michael DeBaun, a hematologist at Vanderbilt University. But it is nonetheless early to say whether or not the procedure will be long term and with out aspect consequences, he adds.
The Food and drug administration also authorized another kind of gene therapy for sickle cell sickness nowadays termed lovo-cel, which is made by the enterprise bluebird bio.
What is sickle cell condition, and how does the new CRISPR remedy get the job done?
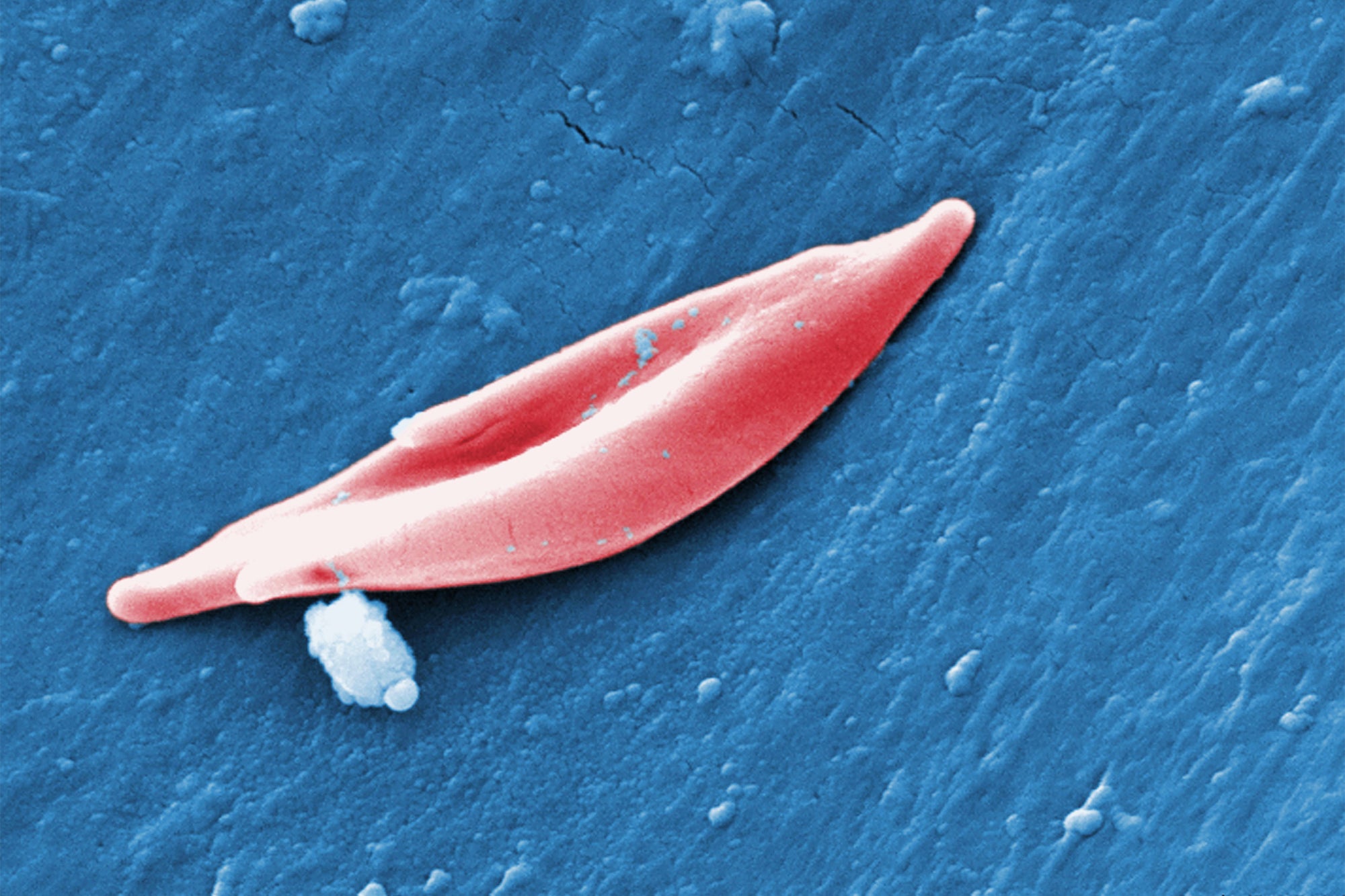
The CRISPR method applied in exa-cel targets genes that create hemoglobin, the protein that carries oxygen in blood cells. In a variety of sickle mobile sickness identified as sickle mobile anemia, mutations in a gene termed HBB have an affect on the protein’s framework, causing it to twist usually spherical purple blood cells into a curved sickle form. These sickled cells clog blood vessels, main to severe ache and tiredness. In a linked issue referred to as beta-thalassemia, which from time to time occurs with sickle mobile anemia, the body does not deliver sufficient hemoglobin or purple blood cells, ensuing in symptoms triggered by minimal blood oxygen degrees. These signs or symptoms consist of tiredness and progress complications in little ones.
Exa-cel directs an enzyme termed Cas9 to a gene, called BCL11A, that normally helps prevent the overall body from producing a kind of hemoglobin located only in fetuses. Cas9 deactivates BCL11A in bone marrow stem cells, exactly where pink blood cells are made, by chopping its DNA, and the cells start creating the fetal hemoglobin and generating crimson blood cells with a ordinary round condition. In the new remedy, doctors clear away a person’s possess bone marrow stem cells, edit them with exa-cel, ruin the rest of the person’s untreated bone marrow and then reinfuse the edited cells.
Because these edited cells sooner or later repopulate the body, exa-cel is regarded a “curative” treatment that will theoretically final the relaxation of the recipient’s everyday living, though Vertex and CRISPR Therapeutics have followed most of their demo individuals for less than two years.
How productive is the procedure?
So much exa-cel has only been tested in about 100 persons with both sickle mobile anemia or beta-thalassemia. Nonetheless, in 2019 the Food and drug administration gave the companies a “fast-track” acceptance that enables it to take a look at the remedy in smaller sized groups of individuals than would generally be necessary.
In these trials, which are however ongoing, 29 of the 30 study individuals with sickle cell anemia had no agony for a single yr in the 18 months subsequent their exa-cel transfusions. And 39 out of 42 beta-thalassemia individuals no lengthier wanted blood or bone marrow transplants—the typical cure for this disease—for one particular yr immediately after the exa-cel intervention. Vertex and CRISPR Therapeutics are continuing to keep track of the remaining members who have not but achieved this time point and will follow all individuals for up to 15 decades.
What are the dangers?
The final results submitted to the Food and drug administration counsel that exa-cel has no key adverse well being impacts, though it can lead to facet consequences this sort of as nausea and fever. But DeBaun factors out that the participants have only been tracked for a brief time and that troubles could crop up later.
Another worry elevated by the Fda is that the Cas9 enzyme could remain energetic and reduce the genome in destinations other than BCL11A, producing so-termed off-concentrate on mutations. The providers modeled the most most likely destinations in the genome the place the enzyme could slice and observed no evidence that this had occurred in demo members. “In light-weight of any new remedy, we continue to be cautiously optimistic,” DeBaun claims.
Moreover exa-cel, what are some other promising therapies for sickle cell illness?
Bluebird bio’s lovo-cel, the other gene treatment that was accredited right now by the Fda, employs a viral vector to deliver a practical model of an adult hemoglobin-developing gene and insert it completely into a person’s genome. The facts bluebird bio submitted to the Food and drug administration confirmed lovo-cel was successful in 36 individuals who ended up followed for a median of 32 months. Dozens of experiments are investigating other varieties of gene therapies for sickle cell anemia and beta-thalassemia that supply regular variations of HBB or other genes to the entire body.
Researchers have found that a approach known as haploidentical transplant could also get rid of sickle mobile anemia. In this treatment method, which is previously commonly applied to treat particular cancers, a person’s bone marrow cells are replaced with individuals from a guardian or sibling who is 50 per cent genetically similar to the receiver but does not have the disease. Benefits that will be offered upcoming 7 days at the American Society for Hematology’s annual convention discovered that 88 p.c of grownups who been given these transplants continued to make usual pink blood cells after two yrs. DeBaun suggests this approach could be notably helpful in reduced- and center-revenue nations around the world because it is probable to price significantly less than gene editing or gene remedy.
Will exa-cel or lovo-cell be offered to anyone with sickle cell illness?
Like most gene enhancing therapies, exa-cel and lovo-cell are probably to be incredibly high-priced. Neither Vertex, CRISPR Therapeutics nor bluebird bio have stated how considerably their respective therapies will value, but estimates counsel that the value for every could be as a great deal as $2 million per affected person. It is nevertheless unclear no matter whether insurers, especially government providers this sort of as Medicaid, will include the therapies or in which scenarios they will do so. Sickle cell disease disproportionately impacts people today of African descent, including African Americans, who are additional probably to have general public coverage as a result of Medicaid than most other groups in the U.S.
DeBaun says that choosing whether to pursue CRISPR gene enhancing or another method this kind of as haploidentical transplant will want to be a shared choice by individuals, their people and their physicians. Even if gene modifying proves to be much more successful at forever curing the illness, it is probable to be a lot less extensively accessible and could just take for a longer period than a transplant from a donor.
Yet, DeBaun claims that exa-cel is a excellent 1st action, and he expects that the technologies will make improvements to as extra is uncovered about CRISPR therapies above the upcoming decade. “This is the to start with mile of a marathon,” he suggests.
[ad_2]
Resource hyperlink


