[ad_1]
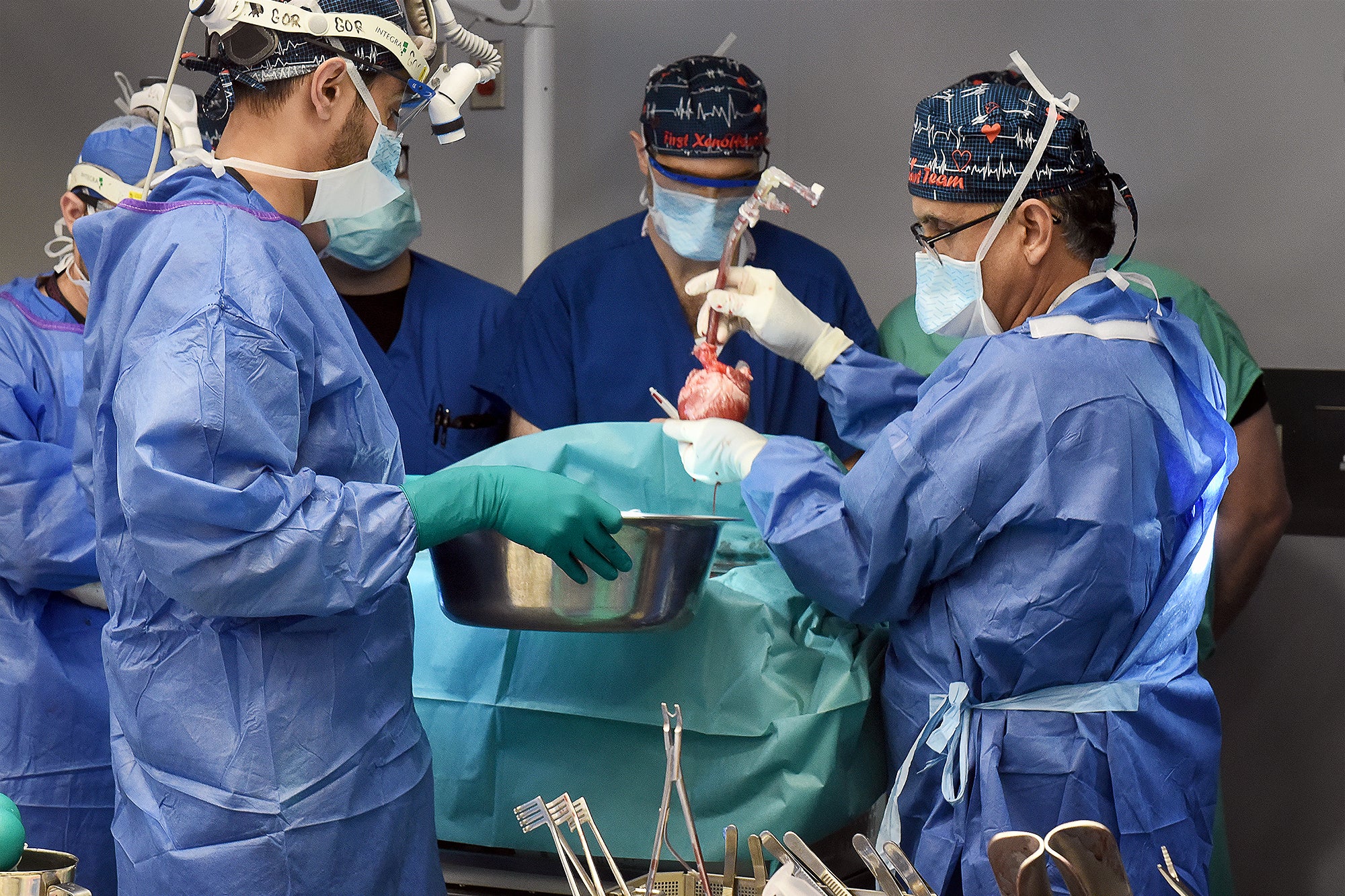
Late past month a staff of scientists at the College of Maryland Faculty of Medicine transplanted a genetically modified pig heart into a person—the next these types of surgical treatment ever attempted—and it has saved him alive for the past couple months. The patient, 58-year-previous Lawrence Faucette, underwent the hugely experimental process below a “compassionate use” pathway, in which the U.S. Foodstuff and Drug Administration permits an unapproved therapy when a individual is critically ill or dying and has no other selections readily available. Faucette was not qualified for a conventional human heart transplant mainly because he experienced peripheral vascular sickness and other difficulties, which narrowed the outlook for achievements.
As of this week, Faucette is continuing to get well and accomplishing bodily treatment. “He’s experienced a rough time,” having said that, claims Bartley Griffith, a surgeon at the University of Maryland, who performed Faucette’s treatment as nicely as the preceding one. In accordance to Griffith, Faucette was living at dwelling when the Food and drug administration initial approved the surgical procedures, but he was subsequently hospitalized with fluid in his lungs. Then he suffered a cardiac arrest the evening just before the surgery. Nonetheless, he has so far responded nicely to the transplant—and was sitting down up in a chair two days afterward. “The upcoming benchmark will be having him up and going for walks,” Griffith claims.
Extra than 100,000 men and women are ready for an organ transplant—most of them for kidneys—so scientists have long been exploring xenotransplantation: transplanting other species’ organs into people. To stop the human immune technique from attacking these alien organs, experts have started to breed genetically modified donor pigs that lack sure genes or have other genes extra.
In the earlier few of years, pig xenotransplants have been examined in the two nonhuman primates and deceased humans—but the top aim is to perform human clinical trials on a even larger scale. The effects of the latest compassionate use transplant will possible impact the FDA’s consideration of irrespective of whether and when to let this sort of trials to get position. Many scientists hope this could take place in the next yr or two.
“I would like to see heart [xenotransplantation in] a medical trial subsequent 12 months and kidney [xenotransplantation trials] shortly thereafter,” states Jayme Locke, director of the division of transplantation at the College of Alabama at Birmingham, who was not involved in the most up-to-date experimental surgical procedure. Locke and her colleagues have executed quite a few kidney xenotransplants in people who experienced endured mind demise. “The Fda holds those cards, and I think it’s heading to genuinely depend on what their risk tolerance threshold is,” she suggests. “But I’m hopeful. I believe the Food and drug administration wants to see this occur.”
In January 2022 Griffith and his team at the College of Maryland transplanted a genetically modified pig heart from the firm Revivicor into a individual, David Bennett, Sr., who lived for two months right before the heart failed, and he passed absent. The heart was afterwards discovered to be contaminated with a pig virus that experienced escaped screening, even though other elements may well have also performed a function in the transplant’s failure and Bennett’s demise.
“We took a fairly superior swing at the ball the very first time, and we got very close to a prolonged results, we feel,” Griffith says. There have been some unforeseen conditions in that very first xenotransplant that may perhaps have influenced its final result, these as the pig virus that was later on identified in the coronary heart, Griffith provides. Considering that then his group and other people have made better methods to exam for these viruses.
Bennett’s household is glad he was equipped to get section. “He lived for two months, and we acquired to commit additional time with him. So I was grateful for that,” suggests his son, David Bennett, Jr., who hopes Faucette’s surgery will be profitable. “I’m thankful for every desire and hope and every man or woman that is involved in this and the capability that it has to shift forward.”
1 significant variation among the initially and 2nd surgical procedures is that even though Faucette was thought of terminally unwell, he was significantly healthier than Bennett was at the time of his treatment. Contrary to Bennett, Faucette experienced been dwelling at property till shortly right before the transplant and was significantly additional cell, according to Muhammad Mohiuddin, director of the Cardiac Xenotransplantation System at the University of Maryland College of Drugs, who is running Faucette’s anti-transplant-rejection regimen.
Other researchers agree that Faucette was a more acceptable candidate for these a novel procedure. “My in general sensation is that this affected person was in significantly far better condition than the former affected individual,” says Nader Moazami, a cardiothoracic transplant surgeon at NYU Langone Well being, who was not concerned in Faucette’s transplant. “Part of the problem when we have a affected individual who is incredibly, pretty sick—and you go into carrying out experimental xenotransplantation, where we nevertheless never necessarily know specifically what mixture of immunosuppressive brokers is good—is that all those sufferers are extremely inclined to building a variety of complications.” Past year Moazami and his colleagues transplanted genetically modified pig hearts into two people who had endured mind loss of life, and the organs functioned very well for quite a few times.
The two Bennett’s and Faucette’s methods used normal immunosuppressive medicines in addition to an experimental a person. With Bennett, the workforce utilized an experimental antibody drug identified as KPL-404, which blocks a receptor named CD40 that activates the host’s B mobile and T mobile immune responses, which can direct to the rejection of a foreign organ. With Faucette, the staff utilized a drug known as tegoprubart, which was developed by Eledon Prescribed drugs and blocks the molecule, or ligand, which binds to CD40. Tegoprubart has been examined in stage 2 clinical trials for human kidney transplants but is not still Food and drug administration-authorised.
The workforce is also working with intercontinental laboratories that are applying artificial intelligence to appraise biopsies of Faucette’s heart tissue, an assessment that Griffith states could detect early indications of tissue rejection.
The Fda will be closely viewing the development of Faucette’s recovery, which could notify the agency’s choice to approve medical trials of xenotransplantation. Locke thinks the 1st trials will probably entail hearts, not kidneys, due to the fact dialysis can maintain men and women with kidney condition alive for a number of years. There is no comparable substitute for coronary heart functionality. Dialysis is nevertheless an imperfect solution, having said that, and Locke hopes kidney xenotransplants will be subsequent. “I consider there is a popular misperception that dialysis is an suitable choice, and it is not,” Locke states. “People might stay a little bit for a longer time on dialysis” than on everyday living-extending heart therapies, she states, but dialysis just cannot switch kidney operate in the extended phrase. Only transplants can do that. “We now have an substitute organ source that I truly imagine is greater than dialysis,” she states. “And it is time to be equipped to examination that.”
[ad_2]
Source link


