[ad_1]
November 17, 2023
4 min study
The research for area-temperature superconductors has endured scandalous setbacks, but physicists are optimistic about the field’s potential
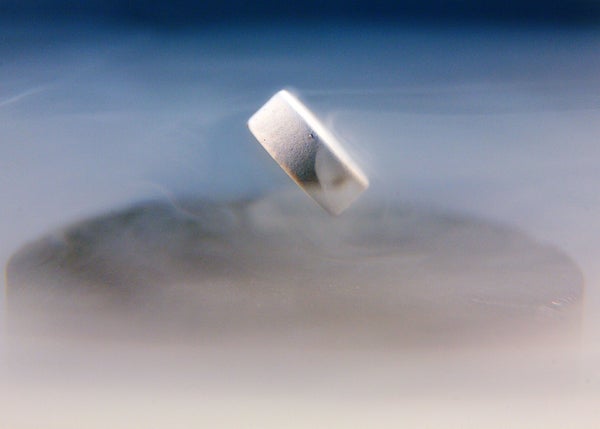
A magnet levitating above a nitrogen-cooled superconductor.
A Nature retraction last 7 days has set to rest the most recent declare of home-temperature superconductivity — in which researchers explained they had manufactured a substance that could conduct electric power without the need of making squander heat and without the need of refrigeration.
The retraction follows the downfall of an even additional brazen declare about a intended superconductor identified as LK-99, which went viral on social media previously this yr.
Irrespective of these large-profile setbacks, superconductivity researchers say the area is making the most of to some degree of a renaissance (see ‘Timeline: Superconductivity milestones’). “It’s not a dying industry — on the opposite,” says Lilia Boeri, a physicist who specializes in computational predictions at the Sapienza College of Rome. The development is fuelled in element by the new capabilities of laptop simulations to forecast the existence and qualities of undiscovered components.
Considerably of the exhilaration is focused on ‘super-hydrides’— hydrogen-loaded resources that have revealed superconductivity at ever-increased temperatures, as extensive as they are kept at large pressure. The subject matter of the retracted Character paper was purported to be these types of a substance, made of hydrogen, lutetium and nitrogen. But work in the previous number of decades has unearthed quite a few people of components that could have innovative houses. “It seriously does glimpse like we’re on the hairy edge of staying ready to locate a whole lot of new superconductors,” claims Paul Canfield, a physicist at Iowa Point out College in Ames and Ames Countrywide Laboratory.
Browsing electrons
Superconductivity occurs when electrons in a solid mix to form ‘Cooper pairs.’ This permits a lot of more electrons than normal to go in sync within the product, which in transform allows the electrons to have currents without generating squander heat.
In ‘conventional’ superconductors, electrons sort Cooper pairs when nudged alongside one another by vibrations in the content — mechanical waves that the Cooper pairs experience like surfers on a wave. Right up until the mid-2000s, researchers normally imagined that this mechanism would function only at exceptionally small temperatures, up to all around 40 kelvin. Superconductors made of a one aspect all need temperatures decrease than 10 kelvin to show this property. Magnesium diboride, a traditional superconductor uncovered in 2001 by a workforce led by Jun Akimitsu at Okayama College in Japan, raised the document for the greatest temperature to 39 kelvin.
The basis for super-hydrides was laid out in 2004, when the late theoretical physicist Neil Ashcroft predicted that certain aspects would variety compounds with hydrogen that could superconduct at much larger temperatures than could any other materials, if place underneath sufficient stress to force the hydrogen atoms nearer alongside one another.
In accordance to Ashcroft’s idea, the proximity of the hydrogen atoms would raise the frequency of mechanical vibrations, which would empower the product to get warmer while retaining its superconductivity. But there was a capture: to even exist, some of these elements would demand pressures equivalent to all those in Earth’s main.
Innovations in carrying out superior-strain experiments on little samples inside a diamond anvil — and measuring their results — led to a breakthrough in 2015, when physicist Mikhail Eremets at the Max Planck Institute for Chemistry in Mainz, Germany, and his collaborators very first demonstrated superconductivity in a tremendous-hydride, hydrogen sulfide. Because then, scientists have predicted the existence of various other superconducting resources in this household — some of which have been located, such as calcium-based cage-like structures referred to as clathrates.
At present, the ‘hottest’ superconductor of any form is viewed as to be lanthanum decahydride, a member of the super-hydride course that is demonstrated to be a substantial-pressure, conventional superconductor at temperatures of up to at minimum 250 kelvin.
Sophisticated simulations
Eremets and other individuals say that the interaction of concept, simulation, elements synthesis and experiment has been very important to progress. Commencing in the early 2000s, it grew to become probable for simulations to forecast whether a material with a particular crystal composition and chemical composition could be a superconductor, and at what temperatures it could exhibit this assets. But the subsequent important shift was the introduction of algorithms afterwards that 10 years that could predict not just the attributes of a content, but what supplies can sort from a specified mix of things. “Until then, a critical bit was missing: comprehending no matter whether a compound can variety in the first area,” claims Boeri.
The discovery in 2015 that hydrogen sulfide is a superconductor was reliable with laptop or computer simulations executed the yr ahead of. With out rapid innovations in structure prediction, the discovery of hydrogen-prosperous superconductors “probably would have not occurred for yet another century,” says Artem Oganov, a supplies scientist at the Skolkovo Institute of Science and Technology in Moscow, who has pioneered construction-prediction algorithms. His ‘evolutionary’ algorithms, in certain, uncover the configuration of atoms with the lowest power — and therefore finest chance to sort and remain secure — at a specified tension.
Simulations are primarily critical for predicting the behaviour of components at significant pressures, below which atoms are pushed so near to a single another that they start off to interact not only by their outer electrons, but also with more interior types, throwing chemistry-textbook dogma out of the window. An example of this is lithium hexahydride, which can exist only at substantial pressures. “Anybody in standard-chemistry course would explain to you that a thing like LiH6 cannot be secure,” states Eva Zurek, a computational chemist at the College at Buffalo in New York.
This post is reproduced with permission and was 1st printed on November 16, 2023.
[ad_2]
Resource connection


